clinical trial malaysia
Ethical issues on Clinical Trial. Novotech Clinical Research Malaysia Suite 2503.

Solving Clinical Trial Delays Three Things To Do Now Pharmaceutical Technology
Within 10 mi 15 km Filter By Advanced I amhavehad ct scan treatment regimen chest x-ray gaa gene movement disorder more I am looking for ergocalciferol etelcalcetide dasatinib pemetrexed adalimumab more Found 202 clinical trials By relevance.
. Overview of GCP and Clinical Research in Malaysia. In addition to issuing licenses for clinical trials conducted in Malaysia NPRA is also responsible for the registration of pharmaceutical products and cosmetics and the enforcement of drug quality control schemes. International Council of Harmonization ICH.
Centre for Clinical Trial CCT was established in 2010 to conduct and support early and late phase clinical research in Malaysia. Since the last publication of Guideline for the application of Clinical Trial Import Licence CTIL and Clinical Trial Exemption CTX 5th Edition in 2009 we have witnessed robust growth in clinical research industry with the aim to achieve at least 1000 clinical trials to generate GNI of RM5784 million by the year 2020 in Malaysia. Featured CROs in Malaysia Parexel.
Hence this second edition guideline is developed in line with the current local regulatory requirements on the manufacture of investigational medicinal products prerequisite of an approved Good Clinical Practice training. National Committee for Clinical Research A committee established for the purpose of coordinating and promoting clinical research in Malaysia chaired by the Director General of Health Ministry of Health. The concerns come as Malaysias.
Clinical Trial Protocol Study Design. Or Europe especially England. List of Clinical Research Organizations in Malaysia Get Listed.
Please confirm that you are not a robot. You have to follow every single phase but first apply for a clinical trial license from NPRA. Ethics and the IRBIEC Overview of Ethics.
NPRA sets some mandatory protocols that you have to follow to get a clinical trial import license in Malaysia. Sponsored clinical studies are usually funded by pharmaceutical companies to conduct clinical trials that involve a research treatment or therapy. If you dont follow these different protocols then you will not be considered eligible to conduct clinical trials for any drugs or medicines.
A clinical trial conducted according to a single protocol but at more than one site and therefore carried out by more than one investigator. 712A GUIDE TO CONDUCTING CLINICAL TRIALS IN MALAYSIA Fig 2. The health spending as a share of Gross Domestic Product GDP for the same period ranged from 295 per cent to 453 per cent of GDP.
On August 2020 the NPRA of Malaysia has updated a document intended to guide the applicant in making Clinical Trial Import Licence CTIL and Clinical Trial Exemption CTX applications to NPRA and reporting to NPRA upon the completion of the clinical trial. Key features of the Malaysian clinical trial landscape include streamlined submission and regulatory processes in English quick start-up times a strong network of experienced KOLs and PIs and supportive Government policies all of which have made Malaysia a preferred clinical trial destination. Singapore is a good location for conducting clinical trials because it boasts the second-best healthcare system in Asia after Japan.
Multiple breaches of standard practices in clinical pharmaceutical trials are raising doubts about the quality of Malaysias medical system and the safety of patients misled into participating in experiments run by doctors accused of gross professional misconduct. Malaysia has inherent benefits to conduct clinical trials such as its large multi-ethnic population that offers genetic diversity Established and good public and private healthcare systems A consistently increasing number of Good Clinical Practice GCP trained and compliant investigators and support staff. Our research facility Clinical Research Ward CRW is.
A listing of Kuala Lumpur Malaysia clinical trials actively recruiting patients volunteers. Hong Kong Indonesia Malaysia the Philippines Singapore Taiwan Thailand and Vietnam In most cases the smaller Asian countries will not require local clinical studies and will accept foreign clinical trial data during the registration process for both medical devices and pharmaceuticals. Offices in this area.
Malaysia Research Clinical Trials RD and Clinical Trials Become a PharmaBoardroom Member for free to access this content Join the 20000 pharmaceutical professionals who already subscribe to PharmaBoardroom. US Clinical Trials Registry. The Health Ministry in Malaysia welcomes applications to conduct clinical trials on products containing cannabis extract for medical purposes as.
In this regard clinical trials conducted with the multi-ethnic Malaysian population can root out different responses to different drugs. In line witht he greater demand of clinical trials in Malaysia and the increasing awareness of GCP amongst our clinicians there is a need to update the current guideline. Furthermore as the below chart shows Malaysia is highly competitive in terms of the costs of conducting clinical research at USD 350 per patient per doctors visit compared to USD 640 in Thailand.
The Total Health Expenditure THE for Malaysia during 1997-2013 ranged from RM8303 million in 1997 to RM44748 million in 2013. In Malaysia sponsored research has been conducted in 220 clinical study sites since 2012 involving Ministry of Health MOH facilities university hospitals and private medical centres. Pursuant to Regulation 29 CDCR 1984 no person is allowed to manufacture supply sell.
EU Clinical Trials Registry. For over 35 years PAREXEL has proven to be a trusted partner for the complex development journey required of biopharmaceutical and medical. GCP Compared to Malaysian Guideline for GCP.
Besides early phase clinical research we also conduct bioavailability and bioequivalence studies for pharmaceutical industries in accordance to local and international standards. Clinical trials are regulated in Malaysia by the National Pharmaceutical Regulatory Agency NPRA an agency under the Malaysian Ministry of Health MOH. We have noticed an unusual activity from your IP 207461386 and blocked access to this website.
Singapore has 43 million people high-quality facilities and highly educated doctors many of whom went to school in the US.

Institute For Clinical Research Icr Nih My Icr Nih Twitter

Introduction To The Clinical Trials Regulation Deloitte Netherlands
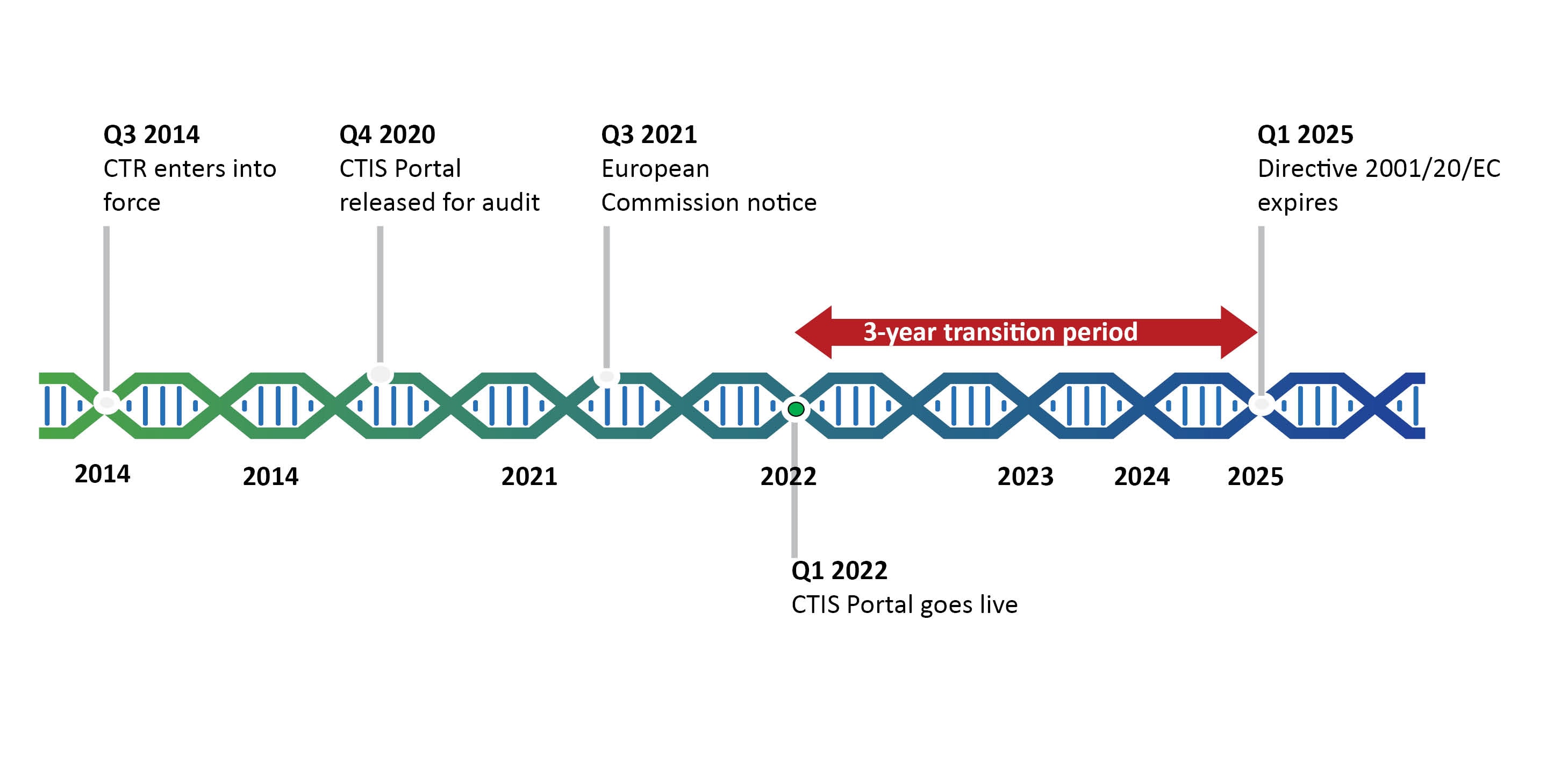
Introduction To The Clinical Trials Regulation Deloitte Netherlands
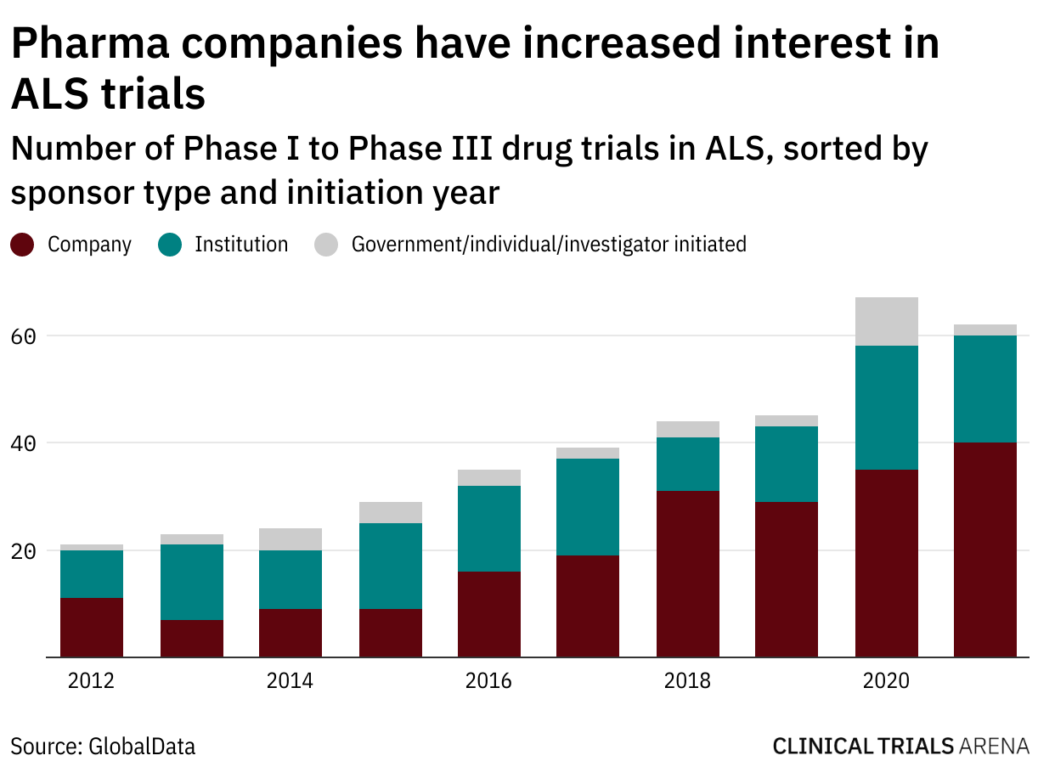
Als In 2022 Most Anticipated Drug Trial Results

Hospital Ampang Clinical Research Malaysia

Home Clinical Update In Covid 19

Why Asia Pacific Is The Next Frontier For Decentralised Clinical Trials Clinical Trials Arena

Cancer Research Malaysia Linkedin

The Future Of Clinical Trials In A Post Covid World Pharmaceutical Technology
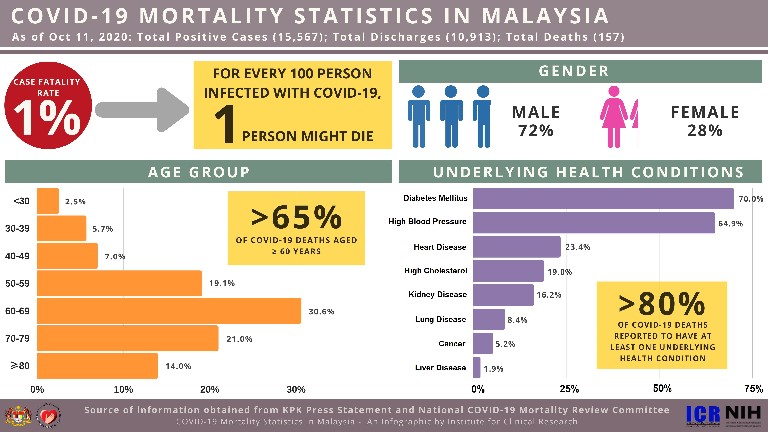
Nih 70 Of Malaysia S Coronavirus Fatalities Had Diabetes Codeblue

Ministry Of Health Malaysia And Drugs For Neglected Diseases Initiative Combine Forces To Lead The Battle Against Dengue Dndi

Hospital Serdang Clinical Research Malaysia

National Medical Research Register

Institute For Clinical Research Icr Nih My Icr Nih Twitter

Home Clinical Update In Covid 19
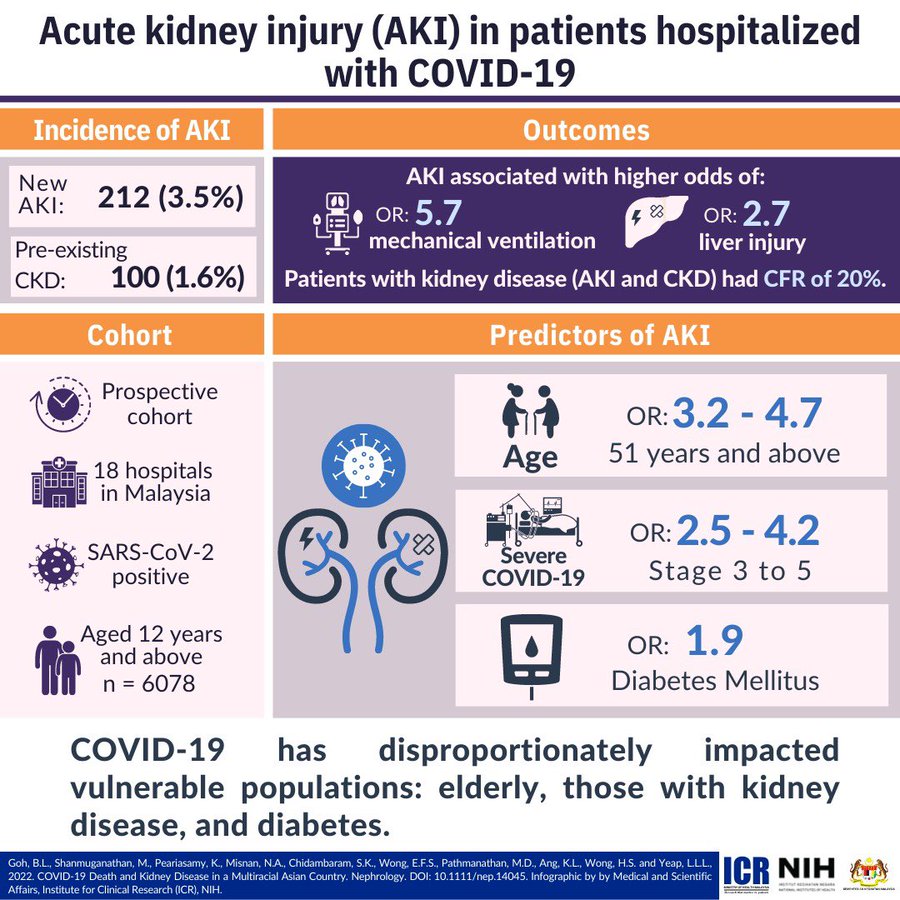
Home Clinical Update In Covid 19

Home Clinical Update In Covid 19

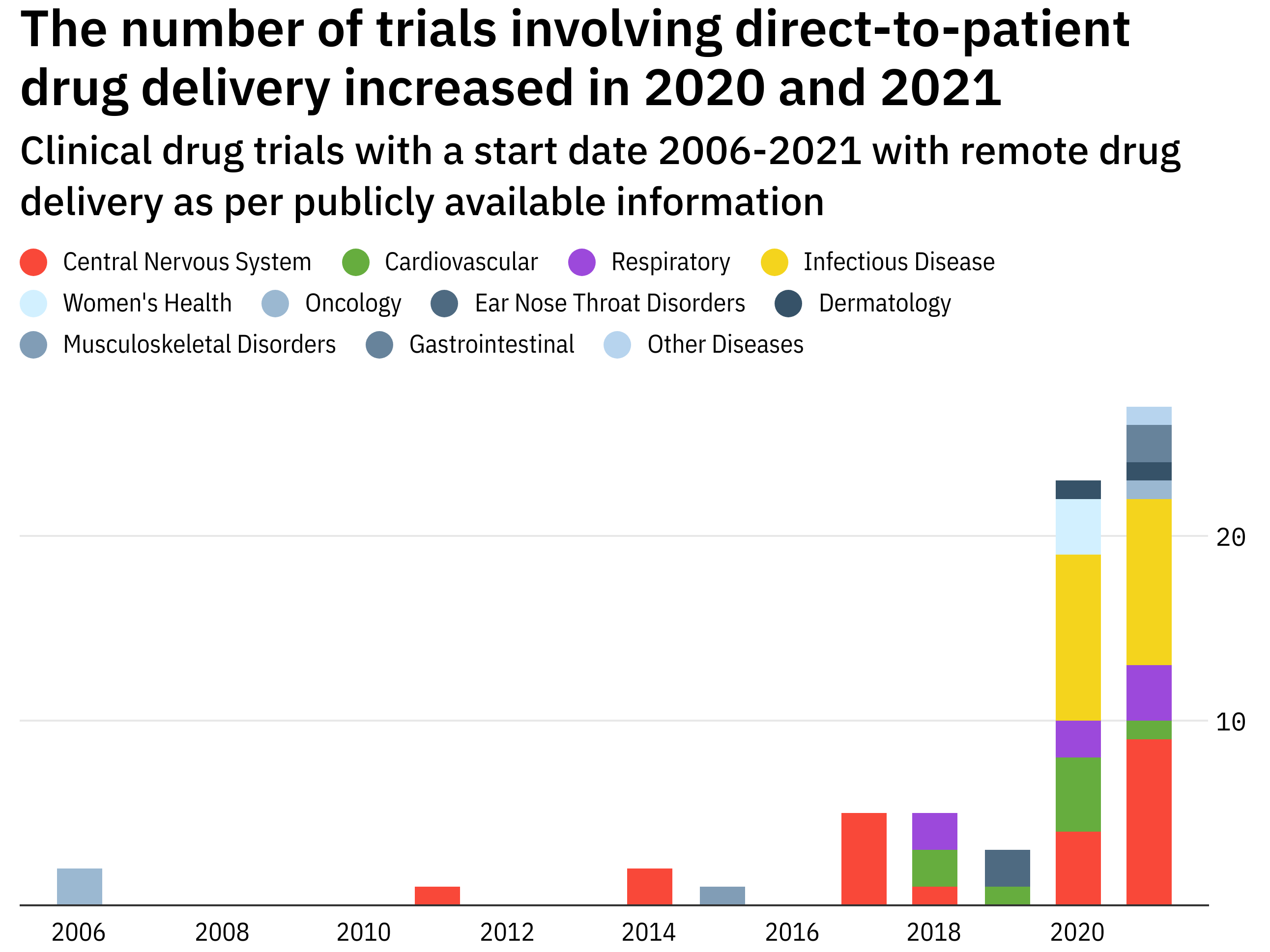
Direct To Patient The Rocky Road To Remote Drug Delivery In Clinical Trials

Comments
Post a Comment